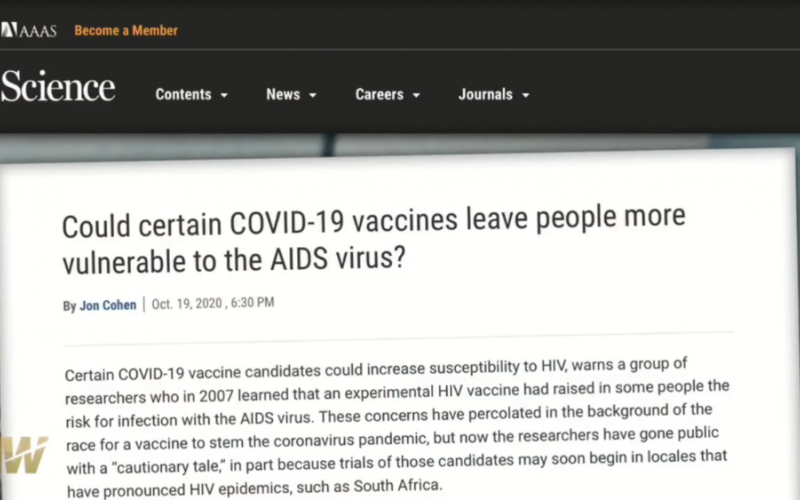
Research article: COVID-19 RNA Based Vaccines and the Risk of Prion Disease, by Dr. J Bart Classen
+ lot of information available in Del Bigtree's video Ep. 194, available on thehighwire.com
references (per the slides, below):
Excerpt from the pdf
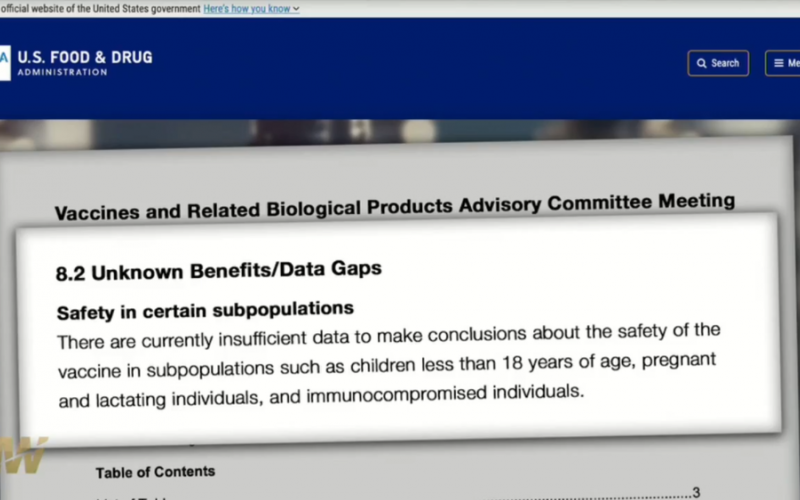
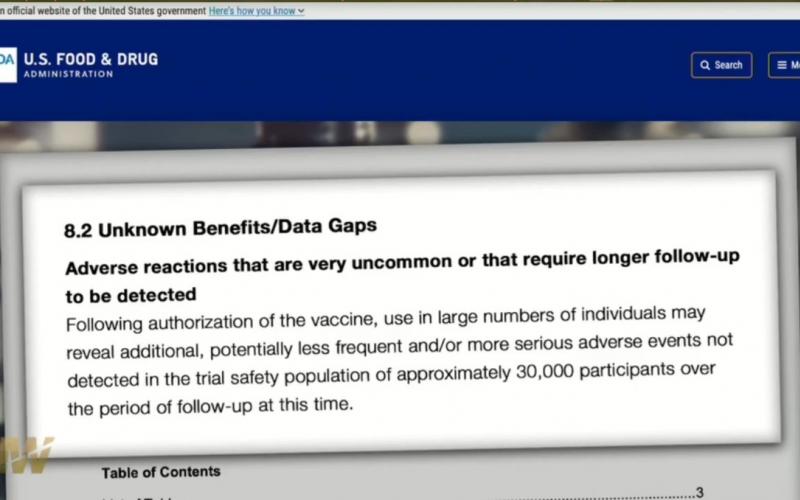
8.2 Unknown Benefits/Data Gaps
Duration of protection
As the interim and final analyses have a limited length of follow-up, it is not possible to assess sustained efficacy over a period longer than 2 months. Effectiveness in certain populations at high-risk of severe COVID-19 Although the proportion of participants at high risk of severe COVID-19 is adequate for the overall evaluation of safety in the available follow-up period, the subsets of certain groups such as immunocompromised individuals (e.g., those with HIV/AIDS) are too small to evaluate efficacy outcomes.
Safety in certain subpopulations
There are currently insufficient data to make conclusions about the safety of the vaccine in subpopulations such as children less than 18 years of age, pregnant and lactating individuals, and immunocompromised individuals.
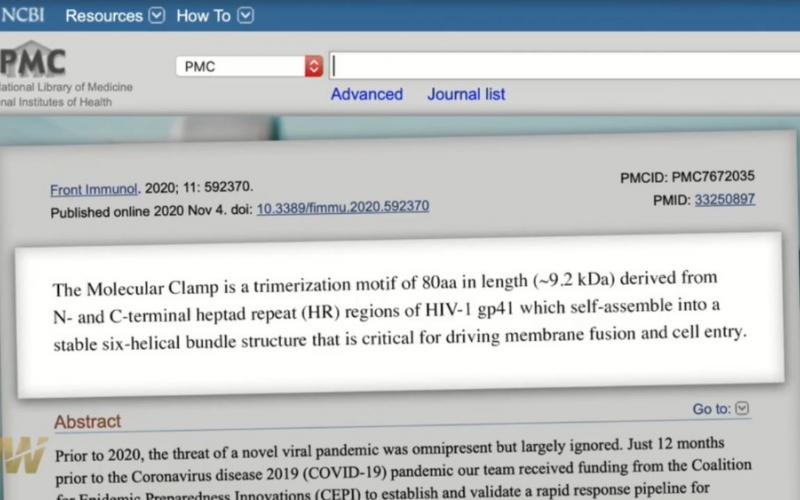
- Rapid Response Subunit Vaccine Design in the Absence of Structural Information (p.2) (US National Library of Medicine)
The Molecular Clamp is a trimerization motif of 80aa in length (~9.2 kDa) derived from N- and C-terminal heptad repeat (HR) regions of HIV-1 gp41 which self-assemble into a stable six-helical bundle structure that is critical for driving membrane fusion and cell entry of HIV-1 (7)
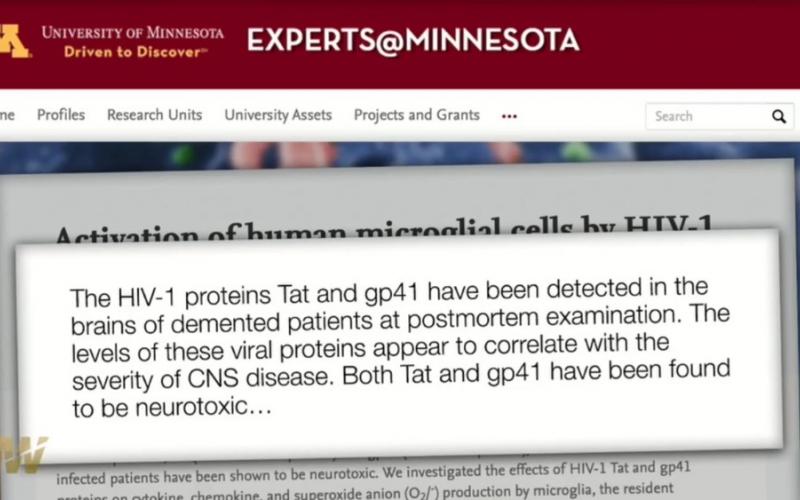
- "gp41 found to be neurotoxic" (paper withdrawn?)
The viral proteins, Tat (HIV-1 nuclear protein) and gp41 (HIV-1 coat protein), detected in the brains of HIV-1-infected patients have been shown to be neurotoxic.


Recent comments